Hepatitis C Treatment Market Set for Significant Growth, Expected to Hit USD 89.9 Billion by 2033
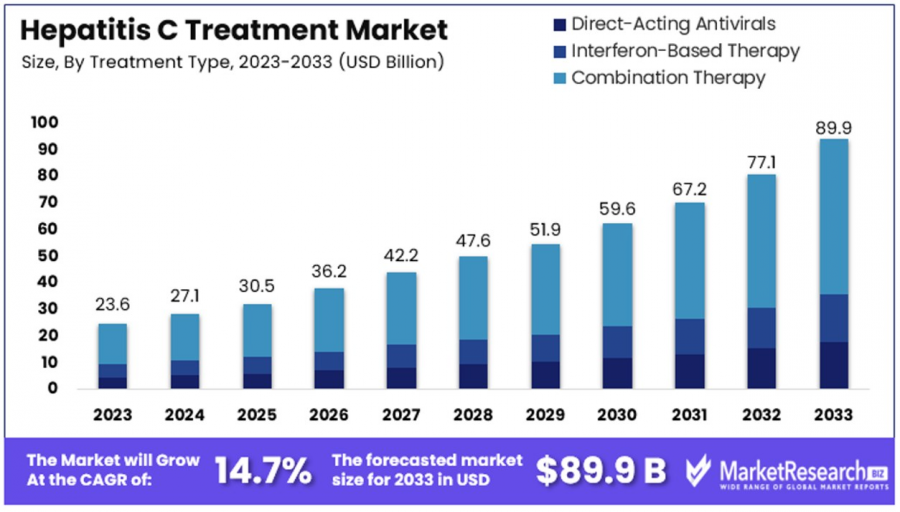
The Global Hepatitis C Treatment Market size is expected to be worth around USD 89.9 Billion by 2033, from USD 23.6 Billion in 2023, growing at a CAGR of 14.7%
NEW YORK, NY, UNITED STATES, February 12, 2025 /EINPresswire.com/ -- Overview
The Global Hepatitis C Treatment Market size is expected to be worth around USD 89.9 Billion by 2033, from USD 23.6 Billion in 2023, growing at a CAGR of 14.7% during the forecast period from 2024 to 2033.
The global fight against Hepatitis C (HCV) is witnessing significant advancements in antiviral therapies, early diagnosis, and patient accessibility. With an estimated 58 million people living with HCV globally, according to the World Health Organization (WHO), effective treatment is critical in reducing disease burden and preventing complications like liver cirrhosis and cancer.
Direct-acting antivirals (DAAs) have revolutionized treatment, offering cure rates above 95% with shorter therapy durations and fewer side effects. These drugs target the virus directly, preventing replication and eliminating HCV from the body. Recent efforts focus on expanding screening programs, affordable generic drugs, and patient support initiatives, improving access to treatment worldwide.
Governments and health organizations are working towards the WHO’s goal of eliminating Hepatitis C by 2030, emphasizing early detection and broader treatment availability. New research also explores pan-genotypic antiviral treatments, effective across all HCV strains, enhancing treatment success rates. With continued innovations and accessibility efforts, Hepatitis C treatment is advancing towards a future of higher cure rates, reduced transmission, and global eradication, improving public health and patient outcomes.
Click here to get a Sample report copy @ https://marketresearch.biz/report/hepatitis-c-treatment-market/request-sample/
Key Takeaways
•Market Value: The global Hepatitis C treatment market is projected to reach USD 89.9 billion by 2033, growing from USD 23.6 billion in 2023 at a CAGR of 14.7% (2024-2033). This growth is fueled by advancements in direct-acting antivirals (DAAs) and expanded treatment accessibility.
•By Treatment Type: Combination Therapy (DAAs + Interferon ± Ribavirin) dominates with a 65.3% market share, driven by its superior efficacy and improved patient outcomes. Interferon-Based Therapy remains relevant in cases where DAAs are unavailable or unsuitable.
•By Stage of Liver Disease: The market is significantly influenced by compensated and decompensated cirrhosis, requiring ongoing treatments. Liver transplantation, though less frequent, remains critical for advanced-stage patients.
•By End-Users: Hospitals lead with a 47.2% market share, offering specialized care and advanced treatment options. Specialty clinics and retail pharmacies also play essential roles in medication accessibility and patient management.
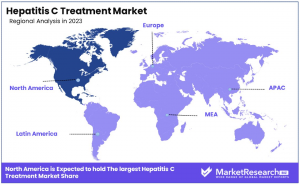
•By Region: North America leads with a 46.2% share, attributed to its advanced healthcare infrastructure and broad treatment accessibility. Europe follows with 25.6%, supported by strong healthcare systems and proactive treatment programs.
•Analyst Viewpoint: The market presents opportunities for further Combination Therapy advancements, focusing on improving efficacy and cost efficiency. Research in decompensated cirrhosis and liver transplantation could drive innovations in treatment strategies and patient outcomes.
Segmentation Analysis
•Treatment Type Analysis: Combination Therapy (DAAs + Interferon ± Ribavirin) dominates the Hepatitis C Treatment Market, accounting for 65.3% of the share. This approach combines Direct-Acting Antivirals (DAAs) with Interferon and Ribavirin, improving viral clearance rates and patient outcomes. While DAAs alone are highly effective, they can be costly, making Combination Therapy a viable alternative. Interferon-Based Therapy, once the standard, now serves a smaller segment, mainly in cases where DAAs are not accessible or suitable for treatment.
•Stage of Liver Disease Analysis: Compensated and decompensated cirrhosis drive the Hepatitis C Treatment Market, requiring continuous medication and disease management. Compensated cirrhosis allows the liver to function despite damage, while decompensated cirrhosis indicates severe impairment, needing intensive care. Liver transplantation, though less frequent, remains critical for patients with irreversible liver damage. Pre- and post-transplant antiviral treatments are essential to prevent HCV recurrence, highlighting the need for specialized medical interventions and ongoing research in advanced liver disease treatments.
•End Users Analysis: Hospitals dominate the market with a 47.2% share, offering comprehensive care, advanced treatments, and specialized monitoring for Hepatitis C patients. They play a crucial role in handling severe cases requiring combination therapies or liver transplants. Specialty clinics focus on hepatology and gastroenterology, providing expert care for chronic cases. Retail pharmacies ensure medication accessibility and adherence, supporting long-term disease management in community settings. Hospitals remain the leading healthcare providers due to their ability to handle complex medical conditions and deliver immediate, specialized treatment.
Market Segments
By Treatment Type
•Direct-Acting Antivirals (DAAs)
•Interferon-Based Therapy
•Combination Therapy (DAAs + Interferon ± Ribavirin)
By Stage of Liver Disease
•Compensated Cirrhosis
•Decompensated Cirrhosis
•Liver Transplantation
By End Users
•Hospitals
•Specialty Clinics
•Gastroenterology Clinics
•Hepatology Clinics
•Retail Pharmacies
To Purchase this Premium Report @ https://marketresearch.biz/purchase-report/?report_id=45393
How Artificial Intelligence (AI) is Changing the Hepatitis C Treatment Market?
•Predicting Treatment Outcomes: AI models are being developed to forecast the success of direct-acting antiviral (DAA) therapies in Hepatitis C patients. By analyzing extensive patient data, these models can identify individuals at higher risk of treatment failure, enabling healthcare providers to tailor interventions more effectively.
•Identifying Patients Lost to Follow-Up: AI-assisted systems have been utilized to locate and re-engage Hepatitis C patients who have been lost to medical follow-up. In a study conducted in Spain, such a system successfully identified and reintegrated patients into the healthcare system, facilitating timely treatment and monitoring.
•Enhancing Diagnostic Accuracy: Machine learning algorithms have been employed to predict Hepatitis C infection and assess liver fibrosis stages by integrating clinical data and blood biomarkers. These AI-based ensemble models have demonstrated higher accuracy compared to traditional methods, aiding in early detection and appropriate treatment planning.
•Personalized Treatment Strategies: AI enables the development of personalized treatment plans by analyzing individual patient characteristics and predicting responses to various therapeutic options. This personalized approach aims to optimize treatment efficacy and minimize adverse effects.
Market Dynamics
••Driver: The introduction of direct-acting antivirals (DAAs) has significantly advanced Hepatitis C treatment. These medications offer high cure rates, shorter treatment durations, and fewer side effects compared to previous therapies. Their effectiveness has led to increased adoption in clinical practice, contributing to the market's growth. For instance, studies have shown that DAAs can achieve sustained virologic response rates exceeding 90%, underscoring their efficacy in eradicating the virus.
••Trend: A notable trend in the Hepatitis C treatment landscape is the expansion of treatment access through policy changes. Some Medicaid programs have lifted prior authorization requirements, facilitating easier access to DAAs for patients. This policy shift has been associated with increased treatment uptake, as evidenced by a study showing that removing such barriers led to a significant rise in the number of patients receiving therapy.
••Restraint: Despite advancements, high treatment costs remain a significant barrier. The expense of DAAs can limit accessibility, especially in low-resource settings. Additionally, insurance denials pose challenges; research indicates that absolute denials of DAA regimens by insurers have remained high and increased over time, regardless of insurance type.
••Opportunity: There is a substantial opportunity to enhance Hepatitis C diagnosis and linkage to care. Implementing simplified diagnostic protocols and point-of-care testing can facilitate earlier detection and treatment initiation. Integrating these approaches into primary healthcare settings can improve patient outcomes and support public health efforts to reduce the Hepatitis C burden.
Market Key Players
•Bristol-Myers Squibb Company
•AbbVie Inc.
•Gilead Sciences Inc.
•Merck & Co., Inc.
•Pfizer Inc.
•F. Hoffmann-La Roche Ltd.
•Teva Pharmaceutical Industries Ltd.
•Roche Holding AG
•Mylan N.V.
•Bayer AG
•Sanofi S.A.
•Vertex Pharmaceuticals Inc.
•Janssen Pharmaceuticals Inc.
•Eli Lilly and Company
•AstraZeneca PLC
Regional Analysis
North America holds a 46.2% share in the Hepatitis C Treatment Market, driven by high disease awareness, advanced healthcare infrastructure, and the presence of major pharmaceutical companies. The region benefits from widespread screening programs, early diagnosis initiatives, and adoption of cutting-edge treatment protocols, strengthening market penetration. Additionally, strong healthcare spending and government support for hepatitis C research and therapy development contribute to sustained growth.
The region's well-structured healthcare system enables effective implementation of treatment strategies, ensuring high patient awareness and access to advanced therapies. The availability of medical insurance and public health initiatives further enhances treatment uptake. With leading pharmaceutical companies actively investing in innovation, North America continues to drive progress in hepatitis C treatment, ensuring the availability of advanced therapeutic options and improving patient outcomes.
Emerging Trends in Hepatitis C Treatment
Recent advancements in direct-acting antiviral (DAA) therapies have significantly improved Hepatitis C treatment outcomes. These oral medications offer cure rates exceeding 95%, shorter treatment durations (typically 8 to 12 weeks), and minimal side effects, making them the preferred choice over older interferon-based treatments.
Efforts to expand access to DAAs are gaining momentum. Policy changes, such as removing prior authorization requirements in some healthcare systems, have facilitated easier access to these effective treatments, leading to increased uptake among patients.
The focus on early diagnosis and treatment initiation is intensifying. Studies suggest that prompt therapy not only improves patient outcomes but also reduces healthcare costs by preventing disease progression to more severe liver conditions.
Use Cases in Hepatitis C Treatment
In clinical practice, DAA regimens have transformed patient care. For example, a study demonstrated that DAA therapy achieved sustained virologic response rates exceeding 90% in patients with chronic Hepatitis C, indicating a high likelihood of cure.
Policy interventions have also shown significant impact. In regions where prior authorization requirements for DAAs were removed, there was a notable increase in treatment uptake, highlighting the importance of healthcare policy in managing Hepatitis C.
These developments underscore the critical role of innovative therapies and supportive policies in enhancing Hepatitis C treatment outcomes and moving towards the goal of disease elimination.
Lawrence John
Prudour
+91 91308 55334
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release