In Vitro Diagnostics (IVD) Market to Hit US$ 167 Billion by 2033
In Vitro Diagnostics Market Size is expected to reach US$ 167.0 billion by 2033 from US$ 84.1 billion in 2023, growing at a CAGR of 7.1%.
NEW YORK, NY, UNITED STATES, January 24, 2025 /EINPresswire.com/ -- The In Vitro Diagnostics (IVD) Market is projected to expand significantly, estimated to grow from US$ 84.1 billion in 2023 to US$ 167.0 billion by 2033, with a compound annual growth rate (CAGR) of 7.1%. This growth is primarily fueled by advancements in precision medicine, which tailors treatments to individual genetic markers and molecular profiles, enhancing diagnostic accuracy and treatment efficacy.
Regulatory changes are also influencing the IVD sector, particularly in the oversight of lab-developed tests (LDTs). Historically under-regulated, these tests are moving towards stricter FDA oversight to ensure their safety and effectiveness, raising industry standards and increasing trust in IVD technologies.
Furthermore, the integration of digital tools and artificial intelligence is revolutionizing IVD systems. These technologies enhance the precision of diagnostic tests by analyzing large data sets and detecting diseases at earlier stages. Automation in laboratory operations is also increasing, reducing errors and improving test throughput.
Lastly, the demand for rapid diagnostic technologies has surged, highlighted by global health crises like the COVID-19 pandemic. This demand is driving innovations in portable diagnostic devices that are simple to use outside traditional lab settings, such as in pharmacies or at home, facilitating faster and more accurate testing. These developments are critical in adapting to emerging health challenges and enhancing accessibility to diagnostic solutions.
Get Sample PDF Report: https://market.us/report/ivd-in-vitro-diagnostic-market/request-sample/
Key Takeaway
• In 2023, the In Vitro Diagnostics market earned $84.1 billion, growing at a 7.1% CAGR, and is projected to hit $167.0 billion by 2033.
• Reagents dominated the product types with a 53.8% market share, compared to instruments and services.
• Molecular diagnostics led the technology segments, capturing 39.5% of the market.
• The infectious disease sector was the largest application area, holding a 44.3% revenue share.
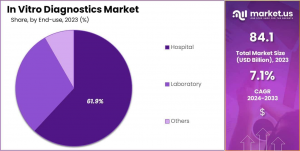
• Hospitals were the primary end-users, accounting for 61.9% of the market revenue.
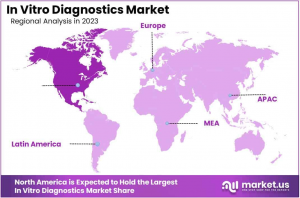
• North America was the market leader with a 39.2% share in 2023.
Segmentation Analysis
In 2023, the reagents segment dominated the market with a 53.8% share, driven by the increasing demand for precise and reliable diagnostic tests. This rise is largely attributed to the growing need for accurate diagnostics to improve patient outcomes, particularly with the escalating prevalence of chronic and infectious diseases. Advances in reagent technology, such as high-throughput assays and multiplex testing, are expected to enhance the efficiency of diagnostics further.
Molecular diagnostics technology claimed a significant market share of 39.5%, fueled by technological advancements that boost testing accuracy and efficiency. The rising demand for personalized healthcare, especially in managing oncology and infectious diseases, supports this growth. Key technologies like polymerase chain reaction (PCR) and next-generation sequencing (NGS) are critical for detecting genetic disorders and pathogens, indicating a robust future for this segment.
The infectious disease application witnessed substantial growth, capturing a 44.3% revenue share. This growth is spurred by the increasing focus on rapid and accurate disease detection through public health initiatives. Innovations in point-of-care diagnostics and multiplex assays are enhancing the speed and accuracy of these tests, crucial in managing the ongoing challenges posed by new and resurgent infectious agents.
Hospitals, as primary centers for patient care, saw significant growth, accounting for 61.9% of the revenue in this sector. This surge is due to hospitals' increasing reliance on advanced diagnostic technologies to improve patient outcomes and operational efficiency. The integration of electronic health records (EHR) and the growing emphasis on early diagnosis are set to further bolster the role of hospital-based diagnostics in the evolving healthcare landscape.
By Product Type
• Reagents
• Instruments
• Services
By Technology
• Hematology
• Immunology
• Coagulation
• Molecular Diagnostics
• Clinical Chemistry
• Microbiology
• Others
By Application
• Diabetes
• Infectious Disease
• Oncology
• Cardiology
• Nephrology
• Drug Testing
• Autoimmune Diseases
• Others
By End-use
• Hospital
• Laboratory
• Others
Regional Analysis
North America has the largest share of the in vitro diagnostics market, capturing 39.2% of the revenue. This dominance is driven by the increasing incidence of chronic diseases and a focus on preventive healthcare. Advanced diagnostic tools are crucial for the early detection and management of health conditions. The market benefits from improvements in laboratory automation and point-of-care testing, which boost diagnostic accuracy and efficiency.
In a notable development, Siemens received FDA clearance in July 2023 for its Atellica CI Analyzer, designed for immunoassay and clinical chemistry. This approval highlights the region's shift toward integrating advanced technologies in laboratories, ensuring healthcare professionals access to precise and timely test results.
The Asia Pacific region is poised for rapid growth in the In Vitro Diagnostics Market, with the highest projected CAGR. Factors fueling this growth include a rise in chronic and infectious diseases and an expanding elderly population. The market's expansion is supported by significant investments in healthcare infrastructure and technology, particularly in India, China, and Japan.
January 2023 saw FUJIFILM Sonosite, Inc. launch the Sonosite PX ultrasound system in India, enhancing clinician ergonomics and operational efficiency. The region's commitment to healthcare advancement is evident, with increased early disease detection awareness and the adoption of home-based testing. These elements are expected to propel the market forward, driven by innovation and improved healthcare access.
Buy Directly: https://market.us/purchase-report/?report_id=96594
Market Players Analysis
The In Vitro Diagnostics (IVD) market is driven by key players who focus on the development and introduction of innovative products. These companies prioritize technological advancement and maintain extensive product portfolios to foster growth. Their commitment to research and development is evident in their investment in next-generation sequencing and point-of-care diagnostics. These technologies enhance the accuracy and speed of diagnostics, key factors in the industry's expansion.
Strategic alliances with healthcare providers and research institutions are crucial for these companies. By forming these partnerships, they enhance their market presence and ensure the adoption of their technologies in clinical settings. This strategy not only expands their operational footprint but also integrates their advanced testing methods into everyday medical practices.
Mergers and acquisitions are common strategies among top IVD companies, aiming to enhance capabilities and integrate complementary technologies. These moves allow them to offer more comprehensive solutions and strengthen their market position. By continuously evolving through acquisitions, these companies can stay ahead of technological advancements and competitive pressures.
Ensuring regulatory compliance and employing robust marketing strategies are also pivotal. These efforts build trust and strengthen relationships with healthcare professionals and patients. Companies like Sysmex Corporation, Hoffmann-La Roche Ltd., Danaher, and Bio-Rad Laboratories are notable for their focus on these aspects, positioning them as leaders in the IVD market.
The Primary Entities Identified In This Report Are:
• Sysmex Corporation
• Hoffmann-La Roche Ltd.
• Danaher
• Bio-Rad laboratories
• Becton Disckinson
• Beckman Coulter
• Arkray
• Abbott Laboratories
• Thermo Fisher
• NeoGenomics
*We offer customized market research reports tailored to meet your specific business needs and requirements.
Lawrence John
Prudour
+91 91308 55334
Lawrence@prudour.com
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release