Preeclampsia Diagnostics Market To Reach USD 1.24 Billion By 2030
Preeclampsia Diagnostics Market Poised for Significant Growth Driven by Rising Awareness and Technological Advancements
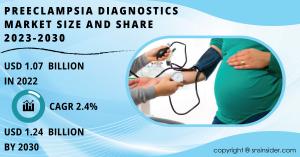
AUSTIN, TEXAS, UNITED STATES, May 6, 2024 /EINPresswire.com/ -- The global market for preeclampsia diagnostics is poised for steady growth, driven by rising awareness about maternal health, advancements in diagnostic technologies, and increasing incidences of preeclampsia. According to SNS Insider, the preeclampsia diagnostics market size was valued at USD 1.07 billion in 2022 and is projected to reach USD 1.24 billion by 2030, with a compound annual growth rate (CAGR) of 2.4% during the forecast period from 2023 to 2030.The preeclampsia diagnostics market report offers a comprehensive analysis of key market trends, drivers, challenges, and opportunities influencing the growth trajectory of the market. It covers various segments such as diagnostic test type, end-user, and region, providing insights into market dynamics and competitive landscape.
Market Analysis
Preeclampsia, a hypertensive disorder during pregnancy, remains a significant concern in maternal healthcare. The demand for accurate and reliable diagnostic tools for early detection and monitoring of preeclampsia has led to the development of innovative diagnostic tests. These tests include biomarker-based assays, imaging techniques, and blood pressure monitoring devices, among others.
The market for preeclampsia diagnostics is driven by factors such as increasing maternal age, rising prevalence of obesity and hypertension, and growing awareness about prenatal care. Healthcare providers are emphasizing the importance of early detection and management of preeclampsia to reduce maternal and fetal complications, thereby fueling the demand for diagnostic solutions.
Download Free Sample Report of Preeclampsia Diagnostics Market @ https://www.snsinsider.com/sample-request/2993
List of Preeclampsia Diagnostics Companies Profiled in Report:
➤ Diabetomics Inc.
➤ PerkinElmer Inc.
➤ F. Hoffmann-La Roche Ltd.
➤ DRG Instruments GmbH
➤ Metabolomic Diagnostics
➤ Thermo Fisher Scientific Holdings Inc.
➤ Sera Prognostics Inc.
➤ Shuwen Biotechnologies Co. Ltd.
➤ Siemens Healthineers AG.
➤ Baxter (U.S.)
Key Highlights from the Report:
➤ By Test Type: The blood tests segment dominated the market in 2022, owing to the widespread use of blood tests for monitoring various biomarkers and detecting preeclampsia at an early stage.
➤ By Product: The equipment segment held the largest market share in 2022, driven by the increasing demand for advanced diagnostic equipment, such as blood pressure monitors and ultrasound machines, for the effective diagnosis and monitoring of preeclampsia.
➤ By End-User: The hospitals segment accounted for the largest market share in 2022, attributed to the high volume of pregnant women seeking prenatal care and the availability of advanced diagnostic facilities in hospital settings.
➤ By Region: North America dominated the preeclampsia diagnostics market in 2022, owing to the presence of well-established healthcare infrastructure, increased awareness about preeclampsia, and the availability of advanced diagnostic tools and techniques.
Regional Analysis:
➤ North America (36.8% market share in 2022): Largest market driven by advanced healthcare infrastructure and high awareness levels
➤ Europe (29.1%): Increasing prevalence of preeclampsia and focus on preventive care fueling growth
➤ Asia Pacific (21.7%): Rapidly expanding market due to improving healthcare access and rising birth rates
Key Developments in the Preeclampsia Diagnostics Market:
➤ In 2023, Roche launched a new biomarker test for early detection of preeclampsia risk during pregnancy
➤ Partnerships between diagnostic companies and research institutes to develop novel preeclampsia screening solutions
➤ Increasing availability of point-of-care testing devices for rapid diagnosis in clinical settings
Key Takeaways from the Preeclampsia Diagnostics Market Study:
➤ Rising incidence of preeclampsia and associated maternal and fetal complications driving demand for early diagnosis
➤ Laboratory tests accounted for the largest segment, offering high accuracy and reliability
➤ Hospitals and prenatal care centers are the major end-users of preeclampsia diagnostic products\
Have Any Query? Ask Our Experts @ https://www.snsinsider.com/enquiry/2993
Emerging Trends and Opportunities
◘ Adoption of Biomarker-Based Tests: Biomarkers such as placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) are gaining traction for their ability to predict preeclampsia risk and severity. Diagnostic tests based on these biomarkers offer improved accuracy and reliability in identifying at-risk pregnancies.
◘ Point-of-Care Testing: The shift towards point-of-care testing is enabling rapid and convenient diagnosis of preeclampsia, particularly in resource-limited settings. Portable devices and rapid test kits are enhancing accessibility to diagnostic services, especially in remote areas.
◘ Integration of AI and Machine Learning: Advanced technologies such as artificial intelligence (AI) and machine learning are being integrated into diagnostic platforms to enhance predictive capabilities and improve patient outcomes. AI-driven algorithms can analyze complex data sets and provide personalized risk assessments for preeclampsia.
Challenges and Considerations
◘ Cost Constraints: The high cost of diagnostic tests and technologies may limit their adoption, especially in developing regions with limited healthcare budgets.
◘ Regulatory Compliance: Stringent regulatory requirements for diagnostic tests, particularly novel biomarker-based assays, can impact market entry and product development timelines.
◘ Awareness and Education: The need for raising awareness among healthcare professionals and pregnant women about the importance of preeclampsia screening and early intervention is crucial for market growth.
Data-driven Recommendations for Market Entry and Growth
◘ Invest in R&D: Continue investing in research and development to develop innovative diagnostic technologies with improved accuracy, affordability, and accessibility.
◘ Collaborate with Key Stakeholders: Establish strategic partnerships with healthcare providers, research institutions, and regulatory authorities to validate diagnostic tests, enhance market access, and ensure compliance with regulatory standards.
◘ Educational Campaigns: Launch educational campaigns targeting healthcare professionals and pregnant women to increase awareness about preeclampsia risk factors, symptoms, and the importance of regular screenings.
◘ Focus on Emerging Markets: Explore opportunities in emerging markets with growing healthcare infrastructure and rising awareness about maternal health, leveraging partnerships and distribution networks to reach underserved populations.
◘ Adopt Digital Health Solutions: Embrace digital health solutions such as telemedicine, remote monitoring, and electronic health records to enhance diagnostic services, improve patient engagement, and facilitate data-driven decision-making.
Purchase Preeclampsia Diagnostics Market Report @ https://www.snsinsider.com/checkout/2993
Other Reports
Hemophilia Market
Mental Health Treatment Market
Akash Anand
SNS Insider Pvt. Ltd
+1 415-230-0044
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Instagram
YouTube
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.